Verification of alkenes with bromine
Chemicals needed
Solution of bromine in carbon tetrachloride T
T  C
C  N R 23/24/25-26-35-40-48/23-52/53-59 S (1/2)-7/9-23-26-36/37-45-59-61
N R 23/24/25-26-35-40-48/23-52/53-59 S (1/2)-7/9-23-26-36/37-45-59-61
cyclohexene F
F  Xn R 11-22 S 9-16-23-33
Xn R 11-22 S 9-16-23-33
toluene F
F  Xn R 11-20 S (2)-16-25-29-33
Xn R 11-20 S (2)-16-25-29-33
The toluene must be pure (free from alkenes, otherwise the verification is useless). One can, of course, analyze other alkenes and instead of toluene use xylene.
Equipment needed
test tube
Test procedure
- Safety goggles! Exhaust hood.
- One adds approx. 1 ml toluene and 2 - 3 drops of the alkene to the test tube.
- Then one adds three drops of the bromine solution and sways the test tube back and forth.
- In a short time the reddish coloring of the bromine becomes weaker, eventually the solution becomes transparent.
Disposal
- The reaction mixture is to be disposed of as halogene-free organic waste.
Elucidation
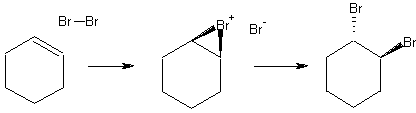
Bromine adds to the double bond of cyclohexene forming trans-1,2-dibromocyclohexane. The reaction can be recognized by the disappearence of bromine coloration.
Photos
to be added
Literature
of own device
